Deadlines for Certification with the American Board of Radiology Approaching Quickly
The American Board of Radiology’s (“ABR”) Board Eligibility Policy, implemented on January 1, 2012, limited the period of time that may elapse between the completion of residency training and achievement of Board Certification. Because a number of radiologists had completed their residencies but not yet achieved Board Certification when the policy went into effect, the ABR established a transitional phase-in period with specific time limits on the Board Eligibility period.
Importantly, the dates chosen by the ABR as the deadlines for achieving certification for certain radiologists are quickly approaching. For diagnostic radiology and radiation oncology, the termination dates for board eligibility status are as follows:
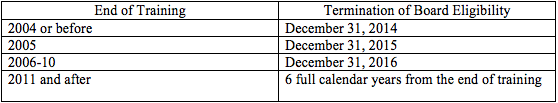
As a result, radiologists who completed their training in 2004 or before but continue in the examination process are facing possible termination of “board eligibility” as soon as the end of this year. After the period of board eligibility expires, radiologists who have not achieved Board Certification will no longer be considered by the ABR to be “board eligible,” and will no longer be permitted to designate themselves as such for credentialing purposes.
 Wachler & Associates Health Law Blog
Wachler & Associates Health Law Blog

